Industry alignment and supporting data qualifying x-ray irradiation of single-use bioprocess equipment as an equivalent alternative to supplement gamma
James Hathcock1.
1Regulatory and Validation Strategy, Pall Corporation, Westborough, MA, United States
Accelerated adoption of irradiated single-use bioprocess systems have been a rapid enabler for production and scale out of vaccines, traditional bioprocessing, continuous bioprocess as well as cell and gene therapies. As security of supply for irradiated systems is critical to patient therapies, key groups within the bioprocess industry have coordinated efforts for a science and risk-based approach to broadly qualify X-ray as an equivalent alternative to supplement gamma irradiation capacity for implementation in 2023. As these highly-customized single-use systems are integrated from multiple components (i.e. filters, tubing, bags) with supporting data packages coming from both component manufacturers and integrators, a successful industry qualification strategy requires strong alignment and openness on the types and level of supporting data that will be generated, shared, and deemed acceptable by regulators, biomanufacturers, integrators, and component manufacturers.
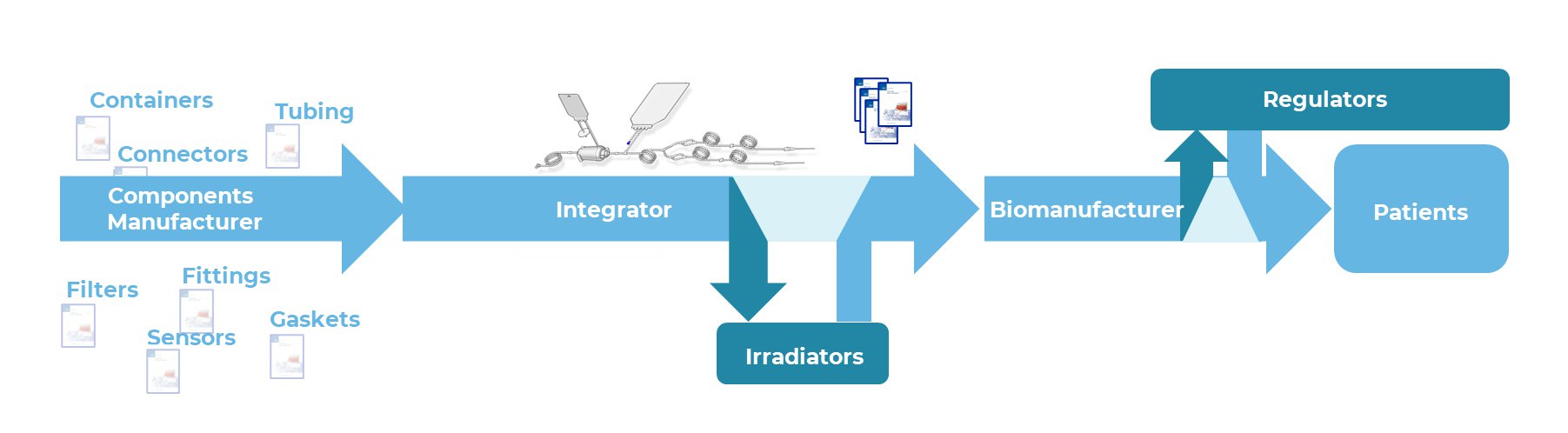
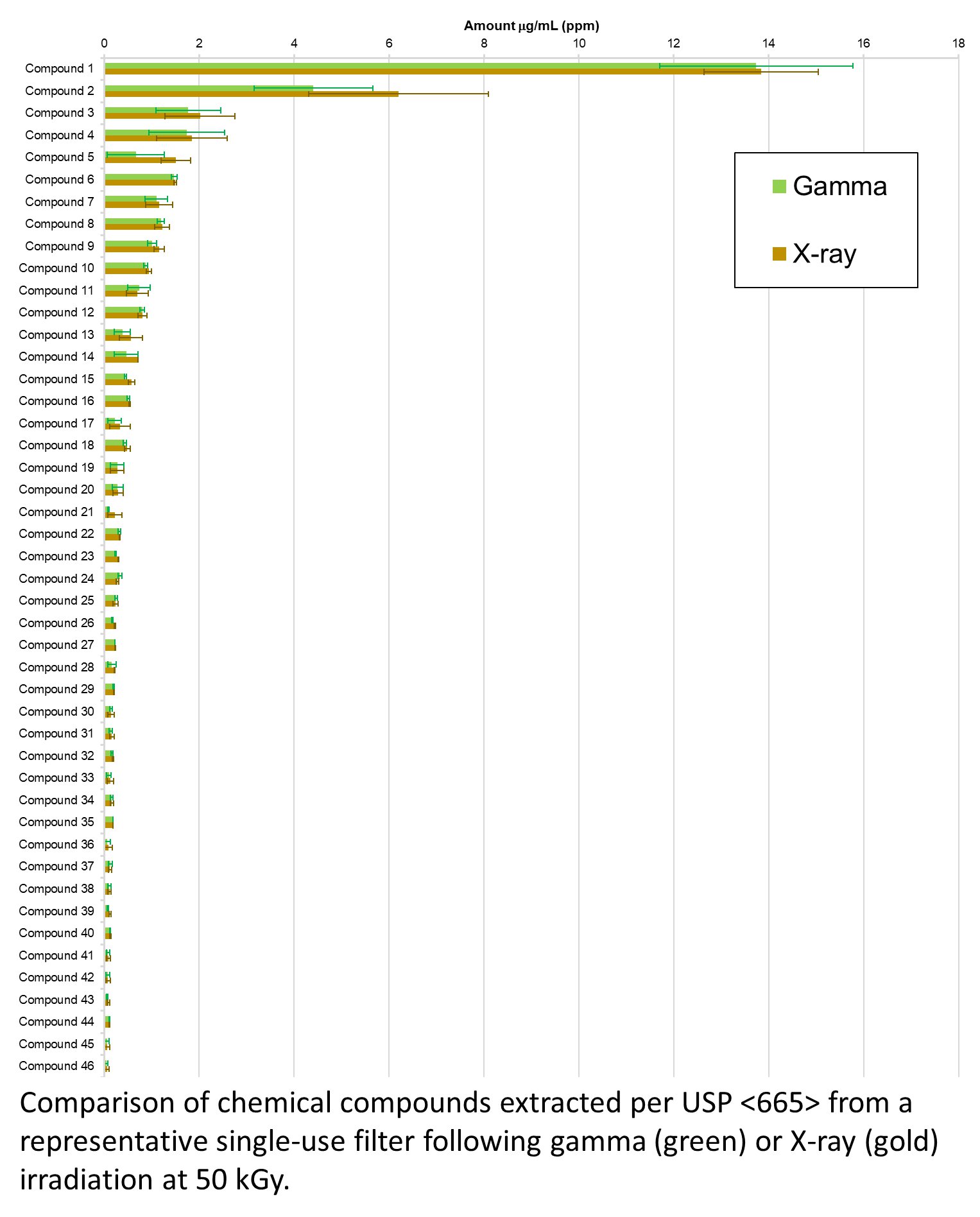
Industry recommendations to qualify X-ray for a single-use system which has previously been qualified for gamma will be highlighted. Examples of supporting data related to transfer of dose from gamma to X-ray will be shared for a broad range of more than 20 representative single-use components (tubing, bags, filters, connectors, mixers) including activation testing, materials assessment (>55 materials assessed), extractables and leachables impact (see figure), functional assessment, and biological reactivity. Key concerns and mitigation strategies expressed by biomanufacturers and regulators will also be summarized.